Press Release
Trellis Bioscience's TRL1068 Receives FDA Fast Track and QIDP Designations, Amplifying Commitment to Combat Infectious Diseases
Redwood City, CA, March 6. 2024. Trellis Bioscience Inc., a pioneering biotechnology company focused on the discovery
and development of native human monoclonal antibodies, is thrilled to announce that its lead antibody candidate, TRL1068, has been granted Fast Track and QIDP
designations by the U.S. Food and Drug Administration (FDA).
Fast Track designation, a prestigious status offered by the FDA, accelerates the development and review of drugs targeting serious conditions with unmet medical needs.
By facilitating early and frequent communication between the FDA and the drug developer, Fast Track designation expedites the delivery of important new therapies to patients.
Additionally, it provides incentives such as priority review and accelerated approval, ensuring that groundbreaking treatments reach those in urgent need as swiftly as possible.
The Fast Track designation typically results in a median reduction of 0.9 years in the approval process. This expedited timeline is expected to bring TRL1068 to patients faster,
addressing critical medical needs more promptly. Driven by a commitment to scientific excellence and patient welfare, Trellis Bioscience is dedicated to advancing the development
of TRL1068 and other innovative therapies to improve outcomes for patients facing serious infectious diseases.
"We are thrilled to receive Fast Track designation for TRL1068, marking a significant milestone in our mission to revolutionize the treatment of infectious diseases," said President & CEO
Stefan Ryser, Ph.D. "This designation underscores the importance of our work in addressing critical medical needs, with a highly innovative approach. We are excited to continue advancing
TRL1068 through clinical development and the regulatory process to bring this innovative therapy to patients in need."
The company has also secured Qualified Infectious Disease Product (QIDP) designation, which incentivizes the development of antibacterial drugs for serious and life-threatening infections.
QIDP designation provides additional benefits to drug developers, including extended market exclusivity and expedited regulatory processes. This designation underscores the critical need for
innovative therapies to combat infectious diseases, particularly in the face of emerging antibiotic resistance.
These designations play a pivotal role in advancing Trellis Bioscience's pipeline, particularly in the development of novel therapies for prosthetic joint infections (PJIs) and other
challenging bacterial infections. The recognition by the FDA underscores the potential of TRL1068 to significantly improve patient outcomes and healthcare delivery.
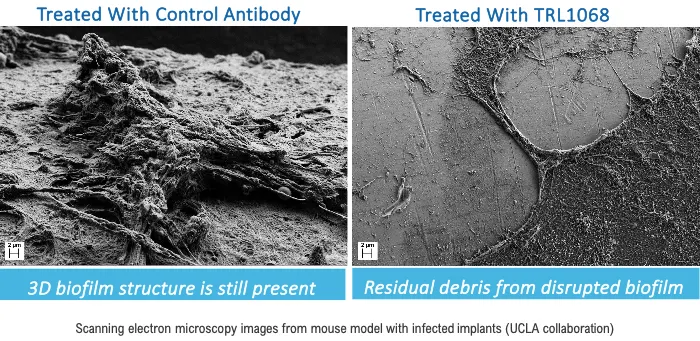
About Prosthetic Joint Infections
The treatment of bacterial infection of implanted medical devices represents a growing unmet need due to the rapidly increasing number of such implants, with over 1
million hip and knee replacements performed each year in the U.S. alone. While these procedures may improve mobility and quality of life, they are associated with complications,
including chronic prosthetic joint infections (PJI). Pathogenic bacteria associated with PJI often form biofilms on the prosthesis, thereby shielding the bacteria from eradication
by antibiotics, resulting in PJI becoming a difficult-to-treat disease. As biofilm cannot be removed by antibiotics, the current standard treatment of chronic PJI is a two-stage
surgical procedure consisting of removal of the infected prosthesis, implant of an antibiotic eluting spacer for several months, and an additional extensive surgical operation to
remove the spacer and implant a new prosthesis. This costly and invasive two-stage process substantially and adversely affects quality of life, with increased immobility and morbidity
in addition to decreased 5-year survival rates in patients with PJI when compared with noninfected patients that receive a joint replacement.
About Clinical Study TRL1068-101:
Clinical Study TRL1068-101 pioneers pharmaceutical research by evaluating TRL1068's potential in treating prosthetic joint infections (PJI) of the knee or hip. This Phase 1 trial employs
a rigorous, double-blinded, randomized, placebo-controlled approach to assess safety, pharmacokinetics, and initial efficacy. Results show promise, as TRL1068 is well tolerated, has no drug-related
adverse events and no dose-limiting toxicity, and with TRL1068 eliminating bacterial biofilm from prosthetic joints within seven days, a groundbreaking achievement. These findings not only reassure
investors but also suggest that TRL1068 could revolutionize PJI treatment, potentially reducing the need for invasive surgeries. Additionally, TRL1068's mechanism, by eliminating biofilm, which allows
traditional antibiotics to work again, hints at broader applications in addressing drug resistance in conditions like bacterial infections in COPD, osteomyelitis, and infective endocarditis, offering
hope for diverse medical needs.
About Clinical TRL1068:
TRL1068 is a native human monoclonal antibody (for intravenous delivery) whose target is a family of bacterial proteins (DNABII) that play a critical role in maintaining the structural
integrity of biofilms by anchoring extracellular bacterial DNA (eDNA) within the biofilm matrix. TRL1068 binds at high affinity (Kd ~50 pM) to a highly conserved epitope found in nearly
all medically significant bacteria, including both Gram-positive and Gram-negative species. Efficacy in animal models for PJI, infective endocarditis, and infections of the lung has been
demonstrated. The International Nonproprietary Names (INN) organization of WHO has granted TRL1068 the generic name calpurbatug. The suffix -tug designates unmodified immunoglobulins (antibodies).
This is the first time that a revised nomenclature scheme for monoclonal antibodies specifically recognizes an unmodified monoclonal antibody.
About Trellis Bioscience Inc.
Trellis Bioscience is a clinical-stage, privately held company focused on discovering and developing native human monoclonal antibodies to treat and prevent drug-resistant,
life-threatening infectious diseases. Antibodies are the immune system's most potent natural weapon against disease. Trellis's innovative proprietary technology CellSpot™
overcomes technical obstacles that have long hindered exploiting the full human antibody repertoire. This ideal source of drugs focuses on the selection of elite antibodies
that bind a target with enormous high affinity from donors who have successfully overcome the disease.
Contact Information
Company: Trellis Bioscience Inc
Address: 702 Marshall Street, Suite 301, Redwood City, CA 94063
Email: info@trellisbio.com
Website: trellisbio.com