Virus Antibody Candidates
Trellis has used its CellSpot technology to identify optimal antibodies from the immune system repertoires of healthy donors. Similar to Trellis' other indication candidates, these antibodies are extremely rare (<1 per 1 million memory B-cells) with high-affinity binding to homologous epitope regions across strain variants. Trellis has developed three antiviral antibody candidates against human cytomegalovirus (HCMV), infuenza, and respiratory syncytial virus (RSV).
Viruses
Cytomegalovirus (CMV) Human mAb, TRL345
Problem Scope
Cytomegalovirus (CMV) is carried by more than half of the population and is the leading cause of serious complications in transplant recipients. CMV infection is a key risk factor in the first 3-6 months post-transplant, affecting 60-70% of patients.
In non-carriers, a CMV infection is a leading non-genetic cause of birth
defects such as hearing loss, visual deficits, and cognitive delays, with an incident comparable to Down's syndrome.
Available antiviral drugs are too toxic for use in both
of these indications, and drug resistance negatively impacts the efficacy for the most commonly used drugs.
CMV costs the US >$2B annually.
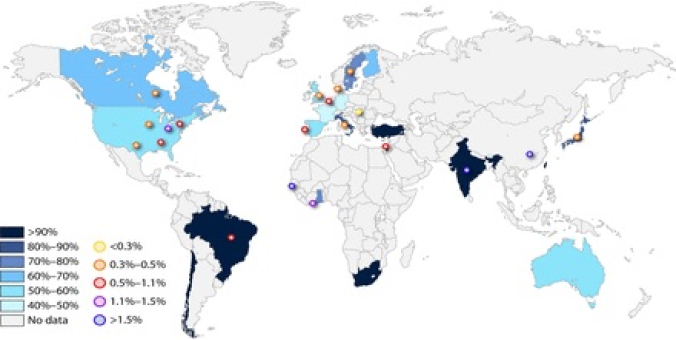
Seroprevalence shaded in blues. Congenital CMV denoted by colored circles
(Source: “’The Silent’ Global Burden of Congenital Cytomegalovirus.”
http://cmr.asm.org/content/26/1/86/F7.expansion.html)

Trellis Innovation
Using CellSpot, Trellis screened over 5 million human memory B-cells (a subset of white blood cells) to discover TRL345, a high-affinity, native human antibody against the most conserved site on a CMV coat protein called glycoprotein B (gB), the surface protein associated with the virus’ entry into human cells. As described in published scientific papers, such antibodies are too rare within the human immune repertoire for reliable discovery by other techniques.


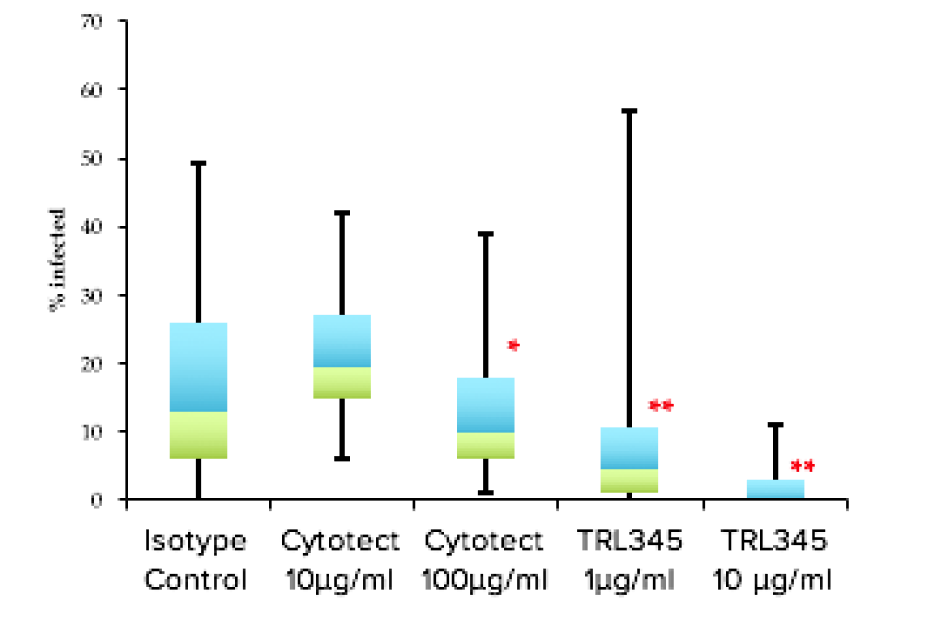
Neutralization studies in vitro showed that TRL345 is 10-fold more potent than previously described anti-gB antibodies and 50-fold more potent than Cytogam (HIG) (right). TRL345 fully neutralized 15 out of 15 clinical isolates, and protected all specialized cell types relevant to the human pathology. It was also fully protective in an ex vivo model of the human placenta grown as a tissue explant.
Since human CMV does not infect animals, making animal models not feasible, this model provides the most compelling support for likely utility in the congenital infection indication.
Viruses
Pan-influenza Human mAb, CF404
Problem Scope
Each year, 5-10% of the US population acquire the flu virus, costing the US $10.4B a year in direct medical expenses (Source: CDC).
On average, more than 200,000 patients within the US are hospitalized each year for seasonal influenza-associated infections.
In 2015, the CDC estimated that influenza vaccination prevented ~67K influenza-associated hospitalizations, but only ~50% of immunized elderly respond to the vaccine and produce neutralizing antibodies.
Emerging pandemic strains, such as the 2009 avian flu which infected 1 billion of the world’s population, remain a threat.


Trellis Innovation
Trellis has leveraged CellSpot's multiplexing assay to develop CF404, an antibody cocktail comprised of 3 mAbs. CF404 targets a region of the influenza virus
that is highly conserved across
multiple influenza strains.
This suggests potential efficacy against
emergent mutant strains.
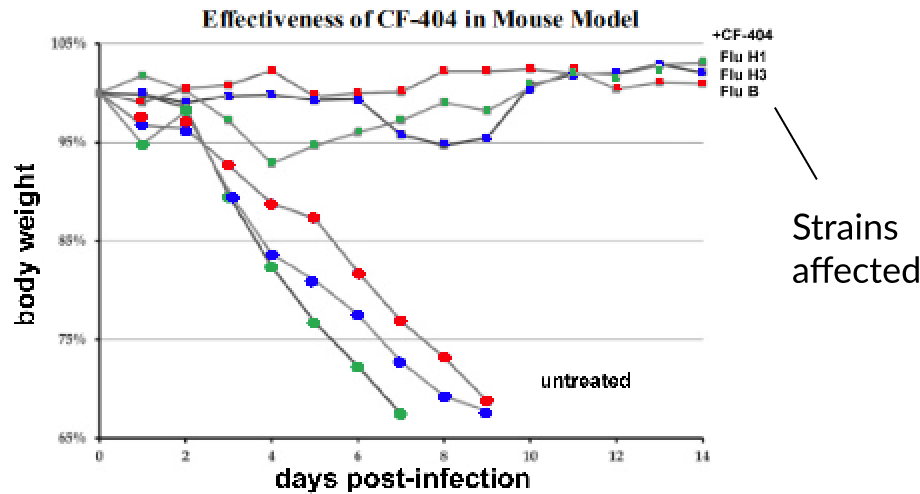
Tamiflu efficacy drops sharply when given more than 24 hours post-infection. CF404 is still highly effective even when given 96 hours post-infection.

Viruses
Respiratory Syncytial Virus (RSV) Human mAb, TRL3D3
Problem Scope
Respiratory Syncytial Virus (RSV) infects nearly all children worldwide by 2 to 3 years of age.
3-7% of children under the age of 2 are hospitalized annually in the US.
Globally, it is a leading cause of lower respiratory tract disease leading to 200,000 deaths and 3 million hospitalizations of young children annually


Trellis Innovation


The currently available antibody treatment, palivizumab (Synagis),
must be administered before infection.
In animal models, TRL3D3, demonstrated 100-fold greater efficacy than palivizumab,
even when administered post-infection.


TRL3D3 is more effective at viral inhibition, demonstrated in a murine model through greater restoration of normal airway function (indicated by lowered airway resistance above) relative to Synagis, even 14 days after infection.